https://www.ncbi.nlm.nih.gov/pubmed/29721153
Abstract
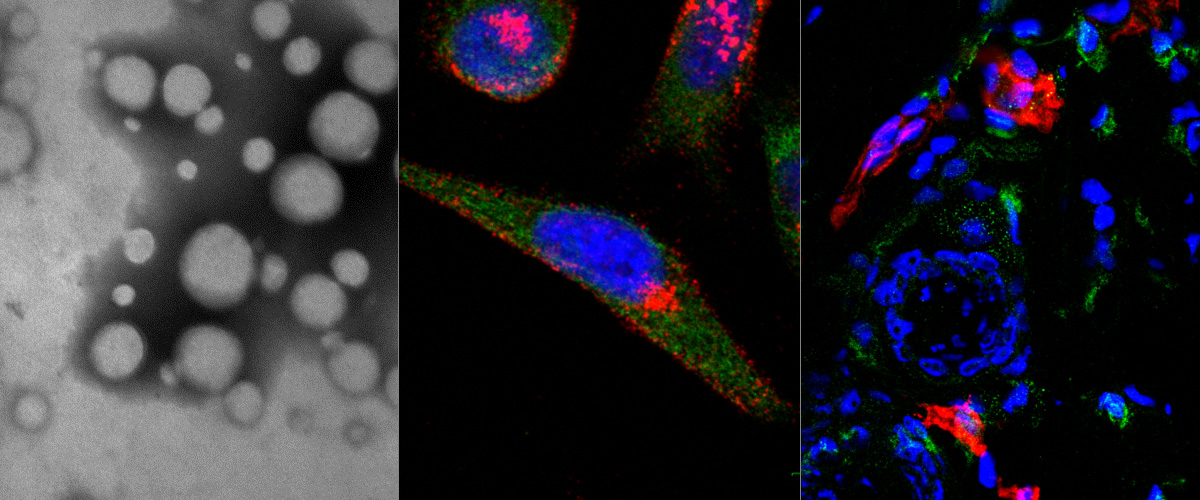
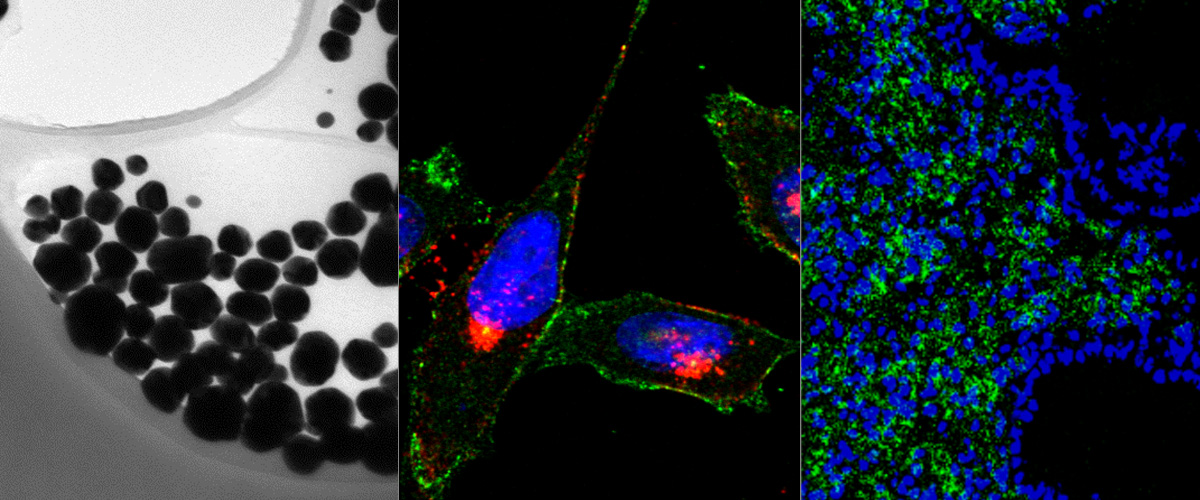
Triple negative breast cancer (TNBC) is the deadliest form of breast cancer and its successful treatment critically depends on early diagnosis and therapy. The multi-compartment protein p32 is overexpressed and present at cell surfaces in a variety of tumors, including TNBC, specifically in the malignant cells and endothelial cells, and in macrophages localized in hypoxic areas of the tumor. Herein we used polyethylene glycol-polycaprolactone polymersomes that were affinity targeted with the p32-binding tumor penetrating peptide LinTT1 (AKRGARSTA) for imaging of TNBC lesions. A tyrosine residue was added to the peptide to allow for 124I labeling and PET imaging. In a TNBC model in mice, systemic LinTT1-targeted polymersomes accumulated in early tumor lesions more than twice as efficiently as untargeted polymersomes with up to 20% ID/cc at 24 h after administration. The PET-imaging was very sensitive, allowing detection of tumors as small as ∼20 mm3. Confocal imaging of tumor tissue sections revealed a high degree of vascular exit and stromal penetration of LinTT1-targeted polymersomes and co-localization with tumor-associated macrophages. Our studies show that systemic LinTT1-targeted polymersomes can be potentially used for precision-guided tumor imaging and treatment of TNBC.