https://www.ncbi.nlm.nih.gov/pubmed/31374479
Abstract
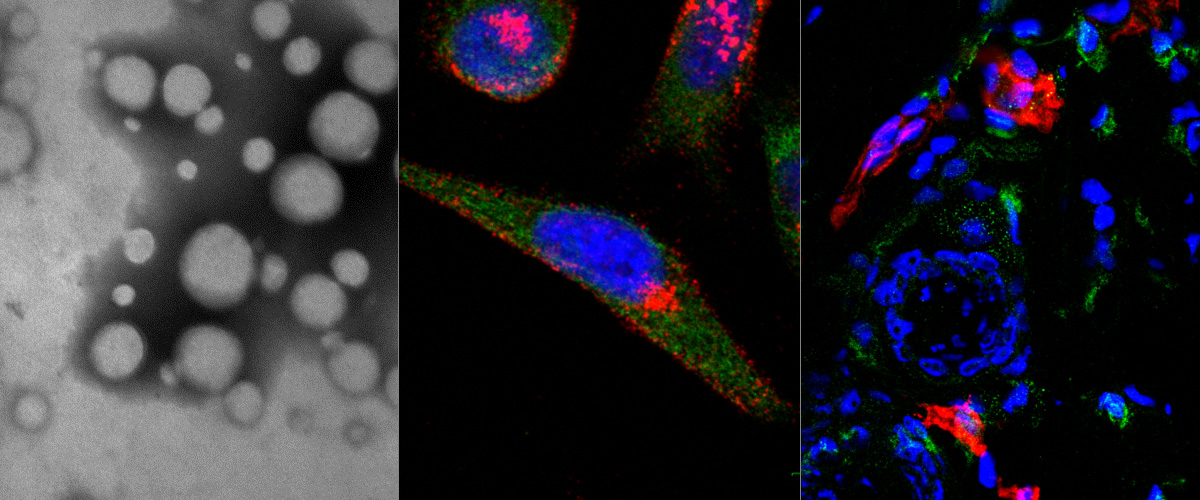
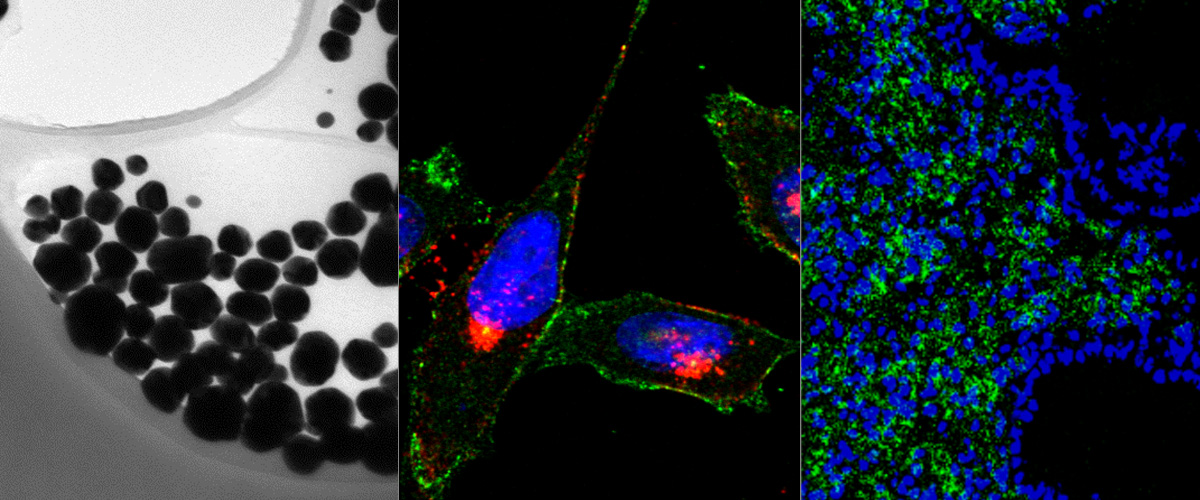
Oncofetal fibronectin (FN-EDB) and tenascin-C C domain (TNC-C) are nearly absent in extracellular matrix of normal adult tissues but upregulated in malignant tissues. Both FN-EDB and TNC-C are developed as targets of antibody-based therapies. Here we used peptide phage biopanning to identify a novel targeting peptide (PL1, sequence: PPRRGLIKLKTS) that interacts with both FN-EDB and TNC-C. Systemic PL1-functionalized model nanoscale payloads [iron oxide nanoworms (NWs) and metallic silver nanoparticles] homed to glioblastoma (GBM) and prostate carcinoma xenografts, and to non-malignant angiogenic neovessels induced by VEGF-overexpression. Antibody blockage experiments demonstrated that PL1 tumor homing involved interactions with both receptor proteins. Treatment of GBM mice with PL1-targeted model therapeutic nanocarrier (NWs loaded with a proapoptotic peptide) resulted in reduced tumor growth and increased survival, whereas treatment with untargeted particles had no effect. PL1 peptide may have applications as an affinity ligand for delivery of diagnostic and therapeutic compounds to microenvironment of solid tumors.