Science day of Department of Biomedicine in Sangaste castle (June 17, 2021)
The research labs of the Department of Biomedicine (Molecular Pathology, Molecular Genetics, RNA Biology, and Precision and Nanomedicine) had a traditional joint Science Day on which graduate students reported on the progress of their projects. The event was held in beautiful Sangaste castle and was full of stimulating talks and Q&A sessions. Presentation by our PhD student Kristina Põšnograjeva “Searching for blood brain barrier penetrating peptides” was voted the winner of the best presentation prize for the best presentation. Congratulations, Kristina!!!
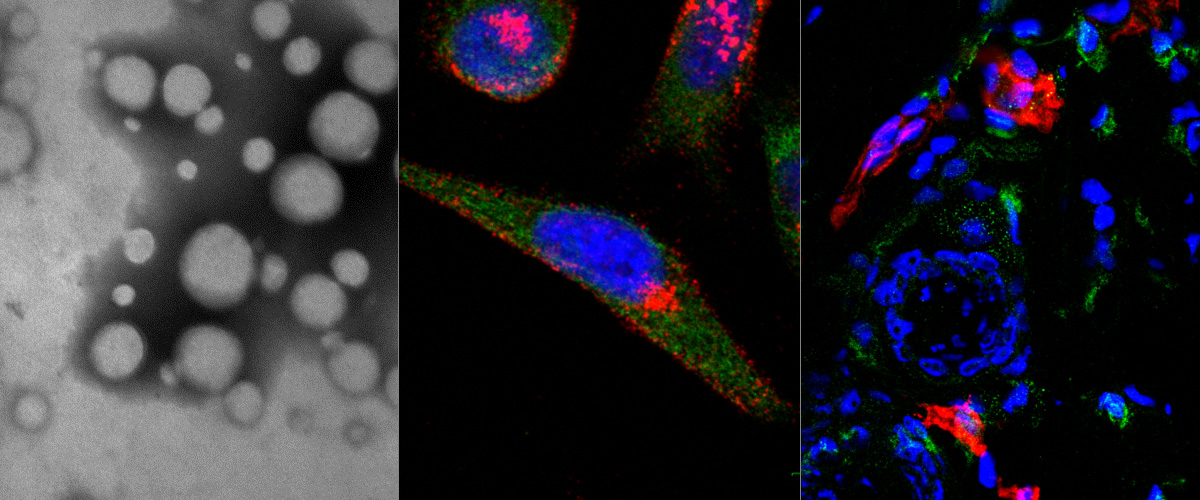
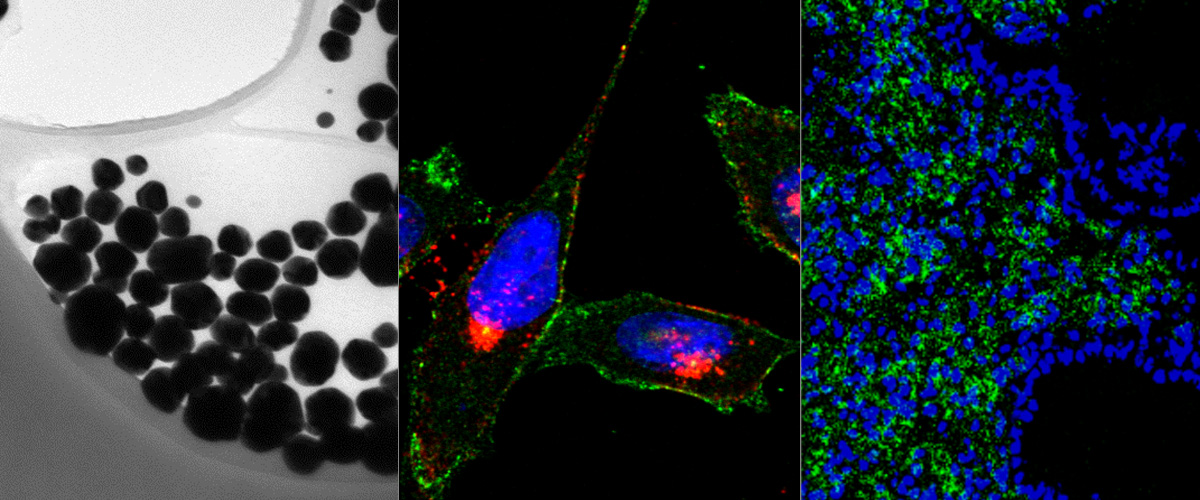
Development of novel anthracycline utorubicin for cancer therapy reported in Angewandte Chemie.
In a study reported in Angewandte Chemie we report the development of Utorubicin (UTO) as a potent anticancer payload of polymeric nanoparticles. Nanoencapsulation of UTO in polymeric vesicles functionalized with tumor-penetrating peptides increases the cytotoxicity of the drug in receptor-positive cells in vitro and, after systemic administration into mice bearing triple-negative tumors, potentiates UTO accumulation in malignant tissue.
This collaborative study was led by team of Precision and nanomedicine (Lorena Simón-Gracia, Valeria Sidorenko, Tambet Teesalu) and involved Estonian drug development company Toxinvent Inc.
Links:
- Novel Anthracycline Utorubicin for Cancer Therapy, Angew Chem, 2021 Jul 26;60(31):17018-17027. https://onlinelibrary.wiley.com/doi/10.1002/anie.202016421
- Nanoparticles Could Help to Deliver Cancer-Drug to the Right Target, https://researchinestonia.eu/2018/12/20/nanoparticles-could-help-to-deliver-cancer-drug-to-the-right-target/
- Smart specialisation project “Innovative anticancer drug candidates based on a novel cytotoxic compound, Utorubicin“
Targeted Delivery of Epidermal Growth Factor to the Human Placenta to Treat Fetal Growth Restriction.
Pharmaceutics. 2021 Oct 25;13(11):1778. doi: 10.3390/pharmaceutics13111778. PMID: 34834193
Abstract
Placental dysfunction is the underlying cause of pregnancy complications such as fetal growth restriction (FGR) and pre-eclampsia. No therapies are available to treat a poorly functioning placenta, primarily due to the risks of adverse side effects in both the mother and the fetus resulting from systemic drug delivery. The use of targeted liposomes to selectively deliver payloads to the placenta has the potential to overcome these issues. In this study, we assessed the safety and efficacy of epidermal growth factor (EGF)-loaded, peptide-decorated liposomes to improve different aspects of placental function, using tissue from healthy control pregnancies at term, and pregnancies complicated by FGR. Phage screening identified a peptide sequence, CGPSARAPC (GPS), which selectively homed to mouse placentas in vivo, and bound to the outer syncytiotrophoblast layer of human placental explants ex vivo. GPS-decorated liposomes were prepared containing PBS or EGF (50-100 ng/mL), and placental explants were cultured with liposomes for up to 48 h. Undecorated and GPS-decorated liposomes containing PBS did not affect the basal rate of amino acid transport, human chorionic gonadotropin (hCG) release or cell turnover in placental explants from healthy controls. GPS-decorated liposomes containing EGF significantly increased amino acid transporter activity in healthy control explants, but not in placental explants from women with FGR. hCG secretion and cell turnover were unaffected by EGF delivery; however, differential activation of downstream protein kinases was observed when EGF was delivered via GPS-decorated vs. undecorated liposomes. These data indicate that targeted liposomes represent a safe and useful tool for the development of new therapies for placental dysfunction, recapitulating the effects of free EGF.
A widespread viral entry mechanism: The C-end Rule motif-neuropilin receptor interaction.
Proc Natl Acad Sci U S A. 2021 Nov 16;118(49):e2112457118. doi: 10.1073/pnas.2112457118. PMID: 34772761
Abstract
Many phylogenetically distant animal viruses, including the new coronavirus severe acute respiratory syndrome coronavirus 2, have surface proteins with polybasic sites that are cleaved by host furin and furin-like proteases. Other than priming certain viral surface proteins for fusion, cleavage generates a carboxy-terminal RXXR sequence. This C-end Rule (CendR) motif is known to bind to neuropilin (NRP) receptors on the cell surface. NRPs are ubiquitously expressed, pleiotropic cell surface receptors with important roles in growth factor signaling, vascular biology, and neurobiology, as well as immune homeostasis and activation. The CendR-NRP receptor interaction promotes endocytic internalization and tissue spreading of different cargo, including viral particles. We propose that the interaction between viral surface proteins and NRPs plays an underappreciated and prevalent role in the transmission and pathogenesis of diverse viruses and represents a promising broad-spectrum antiviral target.
New Tools for Streamlined In Vivo Homing Peptide Identification.
Bi-Functional Peptides as a New Therapeutic Tool for Hepatocellular Carcinoma.
Pharmaceutics. 2021 Oct 6;13(10):1631. doi: 10.3390/pharmaceutics13101631. PMID: 34683924
Abstract
Background: The interfering peptides that block protein-protein interactions have been receiving increasing attention as potential therapeutic tools.
Methods: We measured the internalization and biological effect of four bi-functional tumor-penetrating and interfering peptides into primary hepatocytes isolated from three non-malignant and 11 hepatocellular carcinomas.
Results: These peptides are internalized in malignant hepatocytes but not in non-malignant cells. Furthermore, the degree of peptide internalization correlated with receptor expression level and tumor aggressiveness levels. Importantly, penetration of the peptides iRGD-IP, LinTT1-IP, TT1-IP, and RPARPAR-IP induced apoptosis of the malignant hepatocytes without effect on non-malignant cells.
Conclusion: Receptor expression levels correlated with the level of peptide internalization and aggressiveness of the tumor. This study highlights the potential to exploit the expression of tumor-penetrating peptide receptors as a predictive marker of liver tumor aggressiveness. These bi-functional peptides could be developed for personalized tumor treatment.
Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics.
Pharmaceutics. 2021 Aug 30;13(9):1365. doi: 10.3390/pharmaceutics13091365. PMID: 34575441
Abstract
Cationic liposomes (CLs) are effective carriers of a variety of therapeutics. Their applications as vectors of nucleic acids (NAs), from long DNA and mRNA to short interfering RNA (siRNA), have been pursued for decades to realize the promise of gene therapy, with approvals of the siRNA therapeutic patisiran and two mRNA vaccines against COVID-19 as recent milestones. The long-term goal of developing optimized CL-based NA carriers for a broad range of medical applications requires a comprehensive understanding of the structure of these vectors and their interactions with cell membranes and components that lead to the release and activity of the NAs within the cell. Structure-activity relationships of lipids for CL-based NA and drug delivery must take into account that these lipids act not individually but as components of an assembly of many molecules. This review summarizes our current understanding of how the choice of the constituting lipids governs the structure of their CL-NA self-assemblies, which constitute distinct liquid crystalline phases, and the relation of these structures to their efficacy for delivery. In addition, we review progress toward CL-NA nanoparticles for targeted NA delivery in vivo and close with an outlook on CL-based carriers of hydrophobic drugs, which may eventually lead to combination therapies with NAs and drugs for cancer and other diseases.
P32-specific CAR T cells with dual antitumor and antiangiogenic therapeutic potential in gliomas.
Nat Commun. 2021 Jun 14;12(1):3615. doi: 10.1038/s41467-021-23817-2. PMID: 34127674
Abstract
Glioblastoma is considered one of the most aggressive malignancies in adult and pediatric patients. Despite decades of research no curative treatment is available and it thus remains associated with a very dismal prognosis. Although recent pre-clinical and clinical studies have demonstrated the feasibility of chimeric antigen receptors (CAR) T cell immunotherapeutic approach in glioblastoma, tumor heterogeneity and antigen loss remain among one of the most important challenges to be addressed. In this study, we identify p32/gC1qR/HABP/C1qBP to be specifically expressed on the surface of glioma cells, making it a suitable tumor associated antigen for redirected CAR T cell therapy. We generate p32 CAR T cells and find them to recognize and specifically eliminate p32 expressing glioma cells and tumor derived endothelial cells in vitro and to control tumor growth in orthotopic syngeneic and xenograft mouse models. Thus, p32 CAR T cells may serve as a therapeutic option for glioblastoma patients.
Novel Anthracycline Utorubicin for Cancer Therapy.
Angew Chem Int Ed Engl. 2021 Jul 26;60(31):17018-17027. doi: 10.1002/anie.202016421. Epub 2021 Jun 1. PMID: 33908690
Abstract
Novel anticancer compounds and their precision delivery systems are actively developed to create potent and well-tolerated anticancer therapeutics. Here, we report the synthesis of a novel anthracycline, Utorubicin (UTO), and its preclinical development as an anticancer payload for nanocarriers. Free UTO was significantly more toxic to cultured tumor cell lines than the clinically used anthracycline, doxorubicin. Nanoformulated UTO, encapsulated in polymeric nanovesicles (polymersomes, PS), reduced the viability of cultured malignant cells and this effect was potentiated by functionalization with a tumor-penetrating peptide (TPP). Systemic peptide-guided PS showed preferential accumulation in triple-negative breast tumor xenografts implanted in mice. At the same systemic UTO dose, the highest UTO accumulation in tumor tissue was seen for the TPP-targeted PS, followed by nontargeted PS, and free doxorubicin. Our study suggests potential applications for UTO in the treatment of malignant diseases and encourages further preclinical and clinical studies on UTO as a nanocarrier payload for precision cancer therapy.
Tumor-penetrating therapy for β5 integrin-rich pancreas cancer.
Nat Commun. 2021 Mar 9;12(1):1541. doi: 10.1038/s41467-021-21858-1.
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterized by marked desmoplasia and drug resistance due, in part, to poor drug delivery to extravascular tumor tissue. Here, we report that carcinoma-associated fibroblasts (CAFs) induce β5 integrin expression in tumor cells in a TGF-β dependent manner, making them an efficient drug delivery target for the tumor-penetrating peptide iRGD. The capacity of iRGD to deliver conjugated and co-injected payloads is markedly suppressed when β5 integrins are knocked out in the tumor cells. Of note, β5 integrin knock-out in tumor cells leads to reduced disease burden and prolonged survival of the mice, demonstrating its contribution to PDAC progression. iRGD significantly potentiates co-injected chemotherapy in KPC mice with high β5 integrin expression and may be a powerful strategy to target an aggressive PDAC subpopulation.