Congratulations to Anne-Mari for successfully defending her PhD thesis!
Anne-Mari Anton Willmore successfully defended her Ph.D. thesis „Silver nanoparticles for cancer research“ on November 17th, 2917. Her thesis dealt with the development of silver nanoparticles as a cancer research tool and potential nanocarrier for targeted tumor delivery.
https://www.ut.ee/en/events/anne-mari-anton-willmore-silver-nanoparticles-cancer-research
Image: Anne Mari Anton Willmore and her opponent Professor Hélder A. Santos (D.Sc. Tech.) of the University of Helsinki (Finland) during the thesis discussion.
A peptide homing to early lesions of Alzheimer's disease reported in Nature Communications
The Ruoslahti Lab in La Jolla, in collaboration with our lab, has found a biomarker of early Alzheimer's disease (AD). This biomarker, identified as the protein Connective tissue growth factor (CTGF) is deposited on the brain blood vessels of early AD mice. We have also identified a peptide (named DAG) that recognizes CTGF and homes to early AD mice, well before the appearance of amyloid beta plaques. This peptide can take with it iron oxide nanoparticles that can serve as contrast in Magnetic Resonance Imaging of AD lesions.
Link to the paper: https://www.nature.com/articles/s41467-017-01096-0
Link to the news coverage: Cancer biologists from the University of Tartu help make an important discovery on Alzheimer’s disease
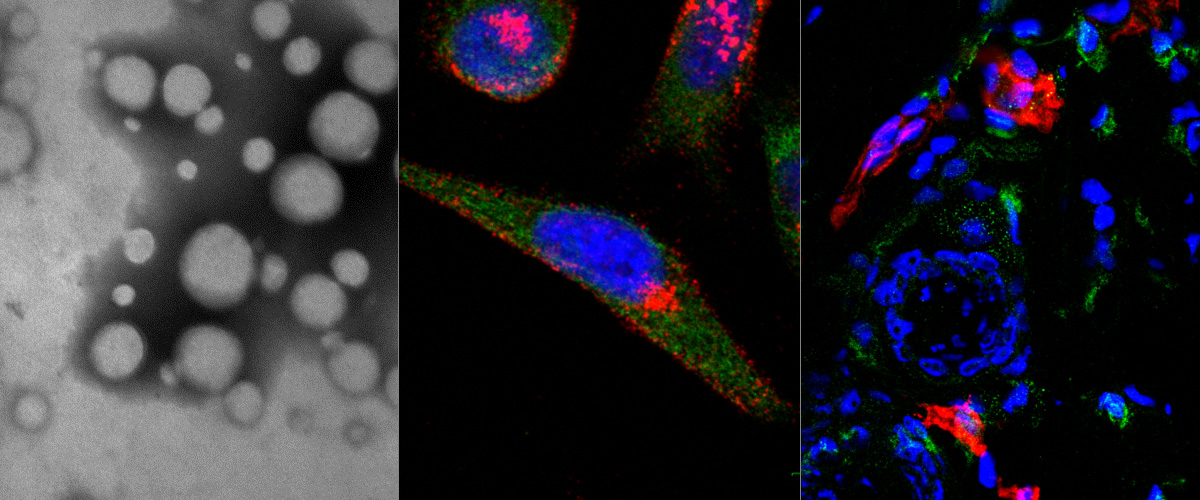
Image: Brain of a mouse with early Alzheimer's disease injected with green fluorescent DAG peptide.. The target protein, CTGF (magenta), appears on the blood vessels (red) of AD brains much before the appearance of amyloid beta plaques. The DAG peptide (green) specifically recognizes CTGF on these blood vessels upon intravenous administration. Credit: Pablo Scodeller and Aman Mann.
Our M2 Tumor macrophage targeting peptide published in Scientific reports!
We have identified a peptide (nicknamed "UNO") that internalizes in M2 tumor associated macrophages (TAMs) by binding to CD206. Systemically administered UNO homed to M2 TAMs in 5 differerent solid tumor models and was able to carry with it a fluorescent payload and nanoscale polymeric vesicles, polymersomes. Importantly, the cyclic UNO peptide preferentially recognizes CD206 in the tumor and not in the healthy tissues, as it is activated by the reducing environment found in the tumor microenvironment.
Link to the paper: https://www.nature.com/articles/s41598-017-14709-x
Image: Cell-free interation of UNO with recombinant CD206. Increase of anisotropy indicates binding of the peptide to CD206. Credit: Pablo Scodeller and Sergei Kopanchuk.
Identification of a peptide recognizing cerebrovascular changes in mouse models of Alzheimer's disease
Mann AP, Scodeller P, Hussain S, Braun GB, Mölder T, Toome K, Ambasudhan R, Teesalu T, Lipton SA, Ruoslahti E.
Nat Commun. 2017 Nov 10;8(1):1403. doi: 10.1038/s41467-017-01096-0.
Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide
Scodeller P, Simón-Gracia L, Kopanchuk S, Tobi A, Kilk K, Säälik P, Kurm K, Squadrito ML, Kotamraju VR, Rinken A, De Palma M, Ruoslahti E, Teesalu T.
Sci Rep. 2017 Nov 7;7(1):14655. doi: 10.1038/s41598-017-14709-x.
Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy
Cureton N, Korotkova I, Baker B, Greenwood S, Wareing M, Kotamraju VR, Teesalu T, Cellesi F, Tirelli N, Ruoslahti E, Aplin JD, Harris LK.
Theranostics. 2017 Aug 29;7(15):3715-3731. doi: 10.7150/thno.19678. eCollection 2017.
Vascular changes in tumors resistant to a vascular disrupting nanoparticle treatment.
Sharma S, Mann AP, Mölder T, Kotamraju VR, Mattrey R, Teesalu T, Ruoslahti E.
J Control Release. 2017 Oct 13;268:49-56. doi: 10.1016/j.jconrel.2017.10.006. [Epub ahead of print]
Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles
Hunt H, Simón-Gracia L, Tobi A, Kotamraju VR, Sharma S, Nigul M, Sugahara KN, Ruoslahti E, Teesalu T.
J Control Release. 2017 Aug 28;260:142-153. doi: 10.1016/j.jconrel.2017.06.005. Epub 2017 Jun 8.
Ratiometric in vivo auditioning of targeted silver nanoparticles
Toome K, Willmore AA; Paiste P, Tobi A, Sugahara KN, Kirsimäe K, Ruoslahti E, Braun GB, Teesalu T.
Nanoscale. 2017 Jul 20;9(28):10094-10100. doi: 10.1039/c7nr04056c.
Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles
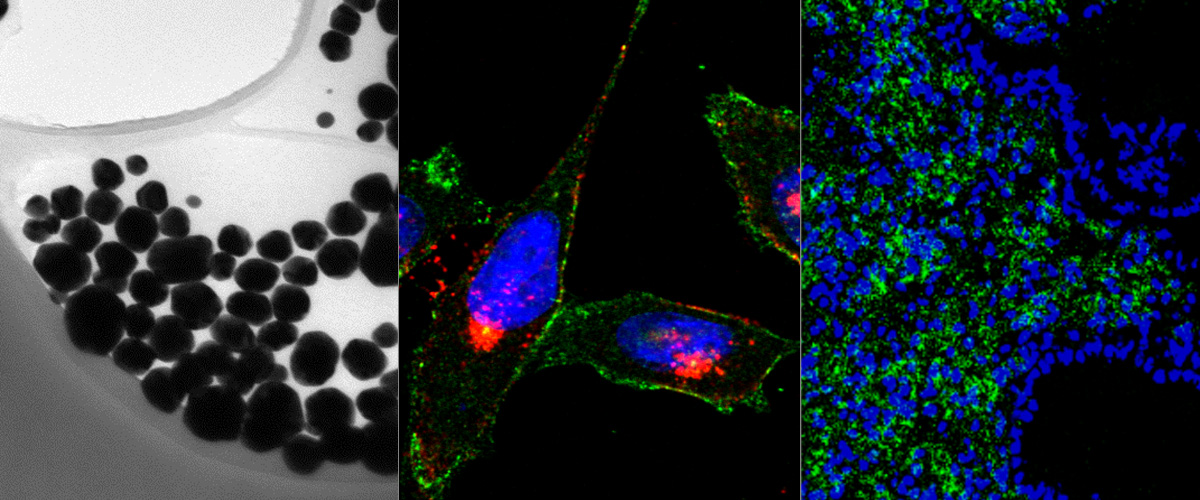
We report in the Journal of Controlled Release application of a novel tumor penetrating peptide, linTT1 (AKRGARSTA) for targeting nanoparticles for the detection and treatment of peritoneal carcinomatosis. Iron oxide nanoworms (NWs) functionalized with the linTT1 peptide were taken up and routed to mitochondria in cultured peritoneal carcinomatosis cells. NWs functionalized with linTT1 peptide in tandem with a pro-apoptotic [D(KLAKLAK)2] peptide showed p32-dependent cytotoxicity in MKN-45P, SKOV-3, and CT-26 cells. Upon IP administration in mice bearing MKN-45P, SKOV-3, and CT-26 tumors, linTT1-functionalized NWs showed robust homing and penetration into malignant lesions, whereas only a background accumulation was seen in control tissues. Finally, experimental therapy of mice bearing peritoneal MKN-45P xenografts and CT-26 syngeneic tumors with IP linTT1-D(KLAKLAK)2-NWs resulted in significant reduction of weight of peritoneal tumors and significant decrease in the number of metastatic tumor nodules, whereas treatment with untargeted D(KLAKLAK)2-NWs had no effect. Our findings suggest that linTT1-targeted nanoparticles may potentially be translated to therapeutic interventions against peritoneal carcinomatosis.
Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles. Hunt H, Simón-Gracia L, Tobi A, Kotamraju VR, Sharma S, Nigul M, Sugahara KN, Ruoslahti E, Teesalu T.J Control Release. 2017 Aug 28;260:142-153. doi: 10.1016/j.jconrel.2017.06.005. Epub 2017 Jun 8. PMID:28603028
Image (Hedi Hunt M.Sc.): Internalized linTT1-NWs colocalize with a mitochondrial marker, cytochrome C in cultured MKN-45P cells. linTT1-NW: green; cytochrome C (Cyt-C): red; DAPI: blue; colocalization of FAM and cytochrome C signal: white. Scale bar: 5 mm.