Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy
Figueiredo P, Lepland A, Scodeller P, Fontana F, Torrieri G, Tiboni M, Shahbazi MA, Casettari L, Kostiainen MA, Hirvonen J, Teesalu T, Santos HA.
Acta Biomater. 2020 Oct 2:S1742-7061(20)30561-4.
doi: 10.1016/j.actbio.2020.09.038 . Online ahead of print. PMID: 33011297
Collaborative study shows that reprogramming tumor macrophages increases the efficacy of chemotherapy in breast cancer mice
Macrophages are immune system cells with a highly plastic phenotype. In established tumors, macrophages are skewed towards a pro-tumoral, anti-inflammatory, phenotype (M2 tumor associated macrophages, or “M2-TAMs”) by tumor-secreted cytokines and hypoxia.
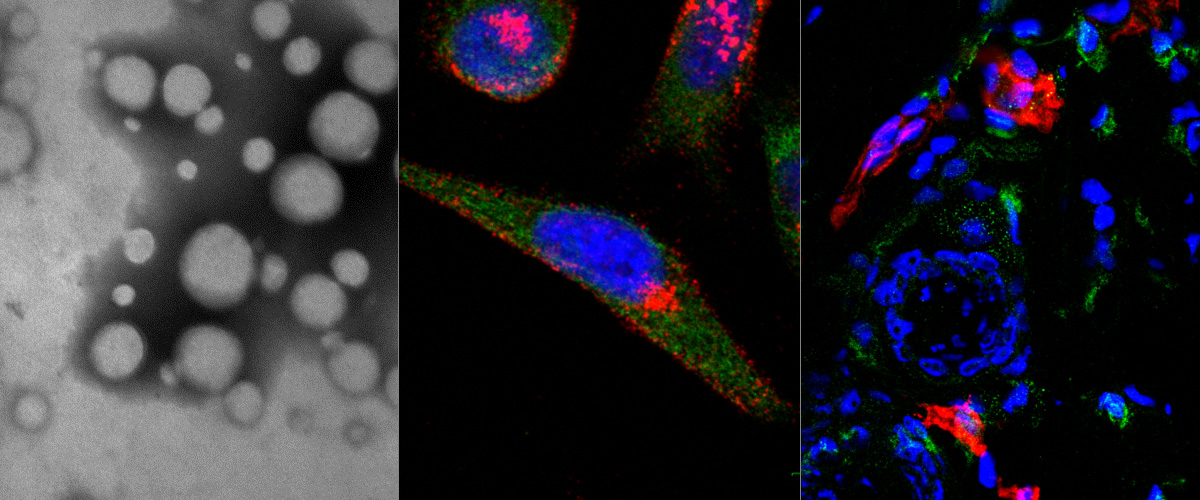
In this collaboration with the Helder Santos Lab (University of Helsinki, Finland), Figueiredo et al. showed that resiquimod was able to enhance chemotherapy in triple negative breast cancer only when it was targeted to M2-TAMs using the peptide "mUNO" identified in our lab. Resiquimod encapsulated in mUNO-coated lignin nanoparticles slowed down the tumor growth and significantly transformed the immune landscape in the tumor: decreased M2-TAM, increased CD8 T-cells, M1-TAM, and IFN-γ; these effects did not occur with free resiquimod or untargeted resiquimod-loaded nanoparticles
Anni Lepland, Pablo Scodeller and Tambet Teesalu from our lab participated in this collaboration, titled: "Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy" https://doi.org/10.1016/j.actbio.2020.09.038
Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug
Tobi A, Willmore AA, Kilk K, Sidorenko V, Braun GB, Soomets U, Sugahara KN, Ruoslahti E, Teesalu T.
Advanced Therapeutics 27 July 2020: 2000097. https://doi.org/10.1002/adtp.202000097. Online ahead of print.
Special issue of Pharmaceutics: “Precision Delivery of Drugs and Imaging Agents with Peptides”
Prof. T. Teesalu and Dr. K. Kurrikoff (Institute of Technology, University of Tartu) act as guest editors of the special issue of Pharmaceutics (IF: 4.4) on peptide-based precision targeting. We invite experts around the world to submit their original research reports and reviews on preclinical and clinical applications of peptide-based targeting for delivery of molecular and nanoscale diagnostic and therapeutic payloads in the context of cancer, cardiovascular and pulmonary diseases, and regenerative medicine.
More information and instructions for submission: https://www.mdpi.com/journal/pharmaceutics/special_issues/precision_delivery
Submission deadline: January 31st 2021.
Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug
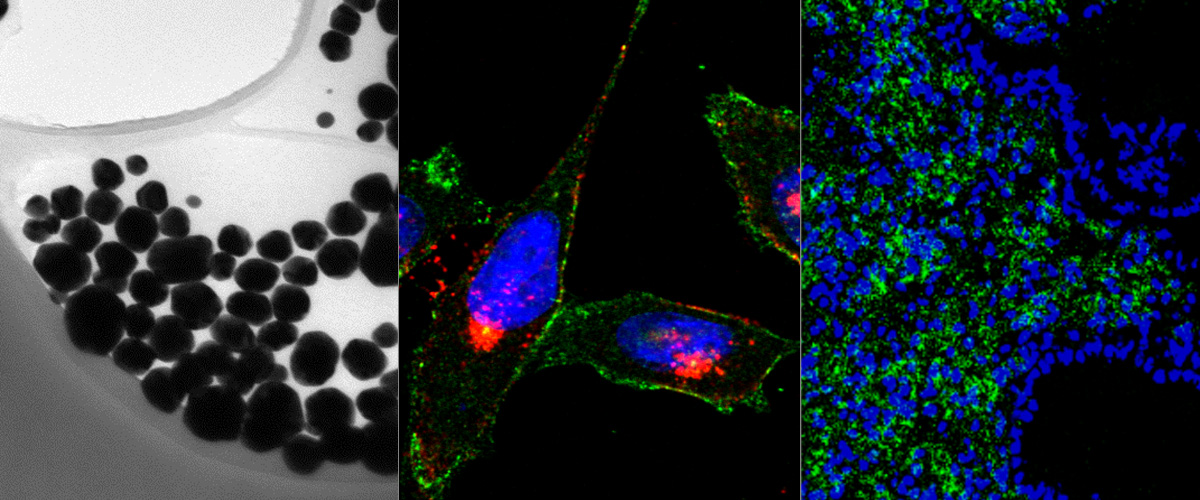
We report application of affinity-targeted silver nanoparticles (AgNPs) for selective delivery and potentiation of the activity of cytotoxic compound, monomethyl auristatin E (MMAE), in malignant cells. AgNPs were loaded with MMAE via a lysosomal protease cathepsin B sensitive linker and functionalized with a prototypic CendR peptide (RPARPAR) that targets neuropilin-1 (NRP-1) to give the AgNPs a dual tumor specificity as both cathepsin B and NRP-1 are overexpressed in many types of solid tumors. Cellular imaging, flow cytometry, viability assays and high performance liquid chromatography-mass spectrometry (HPLC-MS) analysis showed that the RPARPAR-MMAE-AgNPs are internalized and induce apoptotic cell death in NRP-1-positive PPC-1 prostate cancer cells while sparing NRP-1-negative M21 melanoma cells. The anticancer activity of precision target therapeutic AgNPs will be next studied in series of in vivo models, to determine the effect of nanoformulation and affinity targeting on the therapeutic index of MMAE.
This study, published in Advanced Therapeutics, is a sequel to a series of our studies on precision AgNPs. In addition to the contribution of the members of the Cancer Biology team ( PhD cand. Allan Tobi, Dr. Anne-Mari A. Willmore, PhD cand. Valeria Sidorenko and Prof. Tambet Teesalu) the study included contributions of our collaborators in the US (Prof. Erkki Ruoslahti and Ass. Prof. Kazuki N. Sugahara), Canada (Dr. Gary B Braun), and University of Tartu (Prof. Ursel Soomets and Dr. Kalle Kilk).
Journal Title - Advanced Therapeutics Article Title - Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug Manuscript ID - adtp.202000097 https://onlinelibrary.wiley.com/doi/10.1002/adtp.202000097
Uptake of nanoparticles functionalized with prototypic C-end Rule peptide RPARPAR-OH in cultured PPC-1 prostate carcinoma cells (movie: Tambet Teesalu)
Exposed CendR Domain in Homing Peptide Yields Skin-Targeted Therapeutic in Epidermolysis Bullosa
Pemmari T, Ivanova L, May U, Lingasamy P, Tobi A, Pasternack A, Prince S, Ritvos O, Makkapati S, Teesalu T, Cairo MS, Järvinen TAH, Liao Y.
Mol Ther. 2020 May 20:S1525-0016(20)30251-3. doi: 10.1016/j.ymthe.2020.05.017. Online ahead of print. PMID: 32497513
Collaborative studies link CendR pathway to SARS Coronavirus-2 infection and spreading.
Our discovery of the CendR tissue transport pathway in 2009 raised the fascinating question of the physiological function of this pathway. It has become increasingly clear that the CendR pathway has been hijacked by viruses and microbial toxins for cell entry and tissue spreading. Cleavage of a viral surface proteins and protoxins by host proteases (most commonly furins and related enzymes) at sites that create an active CendR motif is a recurrent theme seen in many pathogens. Known examples include the Human T-lymphotropic virus-2,Crimean–Congo hemorrhagic fever virus, tick-born encephalitis virus, and Ebola viruses, as well as anthrax toxin.
In two collaborative studies with laboratories in Finland, Germany and UK; we showed that SARS-CoV-2 Spike protein S1 directly binds to the b1 domain of Neuropilin receptors on the cell surface. These studies also demonstrated that preventing this binding interaction resulted in decreased cell infection. This interaction suggests that neuropilins are important factors that modulate the infectivity of the SARS-CoV-2 viral entry in cells and target for therapeutic approaches to reduce SARS-CoV-2 viral infection.
These findings, published on the preprint server bioRxiv, are undergoing peer review. (http://doi.org/dx5d; 2020) (http://doi.org/dx5c; 2020).
Targeting Pro-Tumoral Macrophages in Early Primary and Metastatic Breast Tumors With the CD206-Binding mUNO Peptide
Lepland A, Asciutto EK, Malfanti A, Simón-Gracia L, Sidorenko V, Vicent MJ, Teesalu T, Scodeller P.
Mol Pharm. 2020 Jun 1. doi: 10.1021/acs.molpharmaceut.0c00226. Online ahead of print. PMID: 32421341
Tumor-penetrating Peptide for Systemic Targeting of Tenascin-C
Lingasamy P, Tobi A, Kurm K, Kopanchuk S, Sudakov A, Salumäe M, Rätsep T, Asser T, Bjerkvig R, Teesalu T.
Sci Rep. 2020 Apr 2;10(1):5809. doi: 10.1038/s41598-020-62760-y. PMID: 32242067
A Novel CNS-homing Peptide for Targeting Neuroinflammatory Lesions in Experimental Autoimmune Encephalomyelitis
Acharya B, Meka RR, Venkatesha SH, Lees JR, Teesalu T, Moudgil KD.
Mol Cell Probes. 2020 Jun;51:101530. doi: 10.1016/j.mcp.2020.101530. Epub 2020 Feb 5. PMID: 32035108