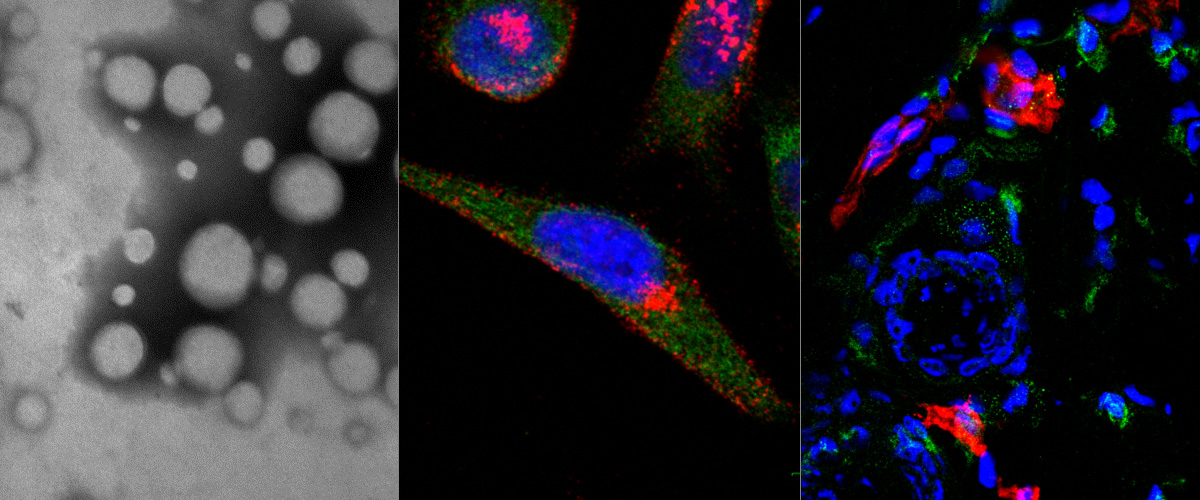
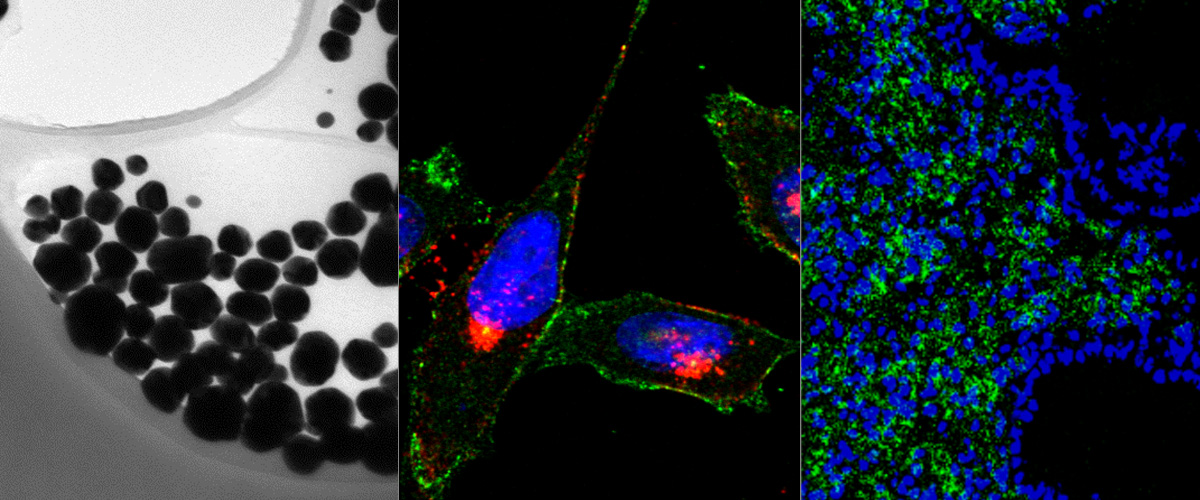
LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy.
Int J Pharm. 2021 Mar 15;597:120346. doi: 10.1016/j.ijpharm.2021.120346. Epub 2021 Feb 2. PMID: 33545283
Abstract
Breast cancer, with around 2 million new cases in 2019, is the second most common cancer worldwide and the second leading cause of cancer death among females. The aim of this work is to prepare a targeting nanoparticle through the conjugation of LinTT1 peptide, a specific molecule targeting p32 protein overexpressed by breast cancer and cancer associated cells, on liposomes' surface. This approach increases the cytotoxic effects of doxorubicin (DOX) and sorafenib (SRF) co-loaded in therapeutic liposomes on both 2D and 3D breast cancer cellular models. The liposome functionalization leads to a higher interaction with 3D breast cancer spheroids than bare ones. Moreover, interaction studies between LinTT1-functionalized liposomes and M2 primary human macrophages show an internalization of 50% of the total nanovesicles that interact with these cells, while the other 50% results only associated to cell surface. This finding suggests the possibility to use the amount of associated liposomes to enrich the hypoxic tumor area, exploiting the ability of M2 macrophages to accumulate in the central core of tumor mass. These promising results highlight the potential use of DOX and SRF co-loaded LinTT1-functionalized liposomes as nanomedicines for the treatment of breast cancer, especially in triple negative cancer cells.
Keywords: Breast cancer; Functionalized-liposomes; Nanomedicines; Targeted therapy: multidrug approach.
Homing Peptides for Cancer Therapy.
Adv Exp Med Biol. 2021;1295:29-48. doi: 10.1007/978-3-030-58174-9_2.
Abstract
Tumor-homing peptides are widely used for improving tumor selectivity of anticancer drugs and imaging agents. The goal is to increase tumor uptake and reduce accumulation at nontarget sites. Here, we describe current approaches for tumor-homing peptide identification and validation, and provide comprehensive overview of classes of tumor-homing peptides undergoing preclinical and clinical development. We focus on unique mechanistic features and applications of a recently discovered class of tumor-homing peptides, tumor-penetrating C-end Rule (CendR) peptides, that can be used for tissue penetrative targeting of extravascular tumor tissue. Finally, we discuss unanswered questions and future directions in the field of development of peptide-guided smart drugs and imaging agents.
Internationally recognized nanomedicine expert Prof. Hélder A. Santos becomes Visiting Professor at our laboratory
With the global benefits of the new science of nanomedicine growing each year, the University of Tartu is determined to stay at the forefront of the international collaborative research in this area.
We are pleased to announce that outstanding researcher, educator, and raising star in nanomedicine, Prof. Hélder A. Santos, joins the Laboratory of Precision and Nanomedicine of University of Tartu as a Visiting Professor (effective February 1st, appointment for 2 years).
Prof Santos is a physical chemist specialized in electrochemistry, biological mimetic models and soft interfaces. His current work bridges engineering, pharmaceutical and medical research. His research focus is in the use of biodegradable and biocompatible nanoporous silicon nanomaterials, polymers, the application of microfluidics technology for nanoparticle production for controlled drug delivery, diagnosis and treatment of cancer, diabetes, and cardiovascular diseases, and further clinical translation of these nanotechnologies. Currently, is a Full Professor in Pharmaceutical Nanotechnology, Head of the Nanomedicines and Biomedical Engineering Group, and Director of the Doctoral Program in Drug Research at the Faculty of Pharmacy, at the University of Helsinki. He is also a Fellow Member of Helsinki Institute of Life Science (HiLIFE), the Director of the FinPharmaNet (The Network of Drug Research Doctoral Programmes in Finland), Chair of Controlled Release Society Focus Group in Nanomedicine and Nanoscale Delivery, and Chairman and co-founder of Capsamedix Oy.
The involvement of Prof. Santos will further strengthen the collaborative ties between the research labs and institutions to further facilitate collaborative efforts in translational nanomedicine and training of next generation of nanomedicine experts.
https://scholar.google.com/citations?user=K3Pj_gwAAAAJ&hl=fi
Contact info: This email address is being protected from spambots. You need JavaScript enabled to view it.
In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues
Pleiko K, Põšnograjeva K, Haugas M, Paiste P, Tobi A, Kurm K, Riekstina U, Teesalu T.
Nucleic Acids Res. 2021 Jan 14, Online ahead of print. PMID: 33444445.
DOI: 10.1093/nar/gkaa12795. Epub 2020 Oct 20
New tools to increase the reliability of in vivo phage biopanning reported in Nucleic Acids Research
In vivo phage display is widely used for the identification of organ- or disease-specific homing peptides. In our study (lead authors PhD students Karlis Pleiko and Kristina Põšnograjeva), we developed new tools to streamline in vivo biopanning screens. Our approach allows differential mapping of homing ability and organ-selectivity of peptide-displaying phages during in vivo selection based solely on the high throughput sequencing data. This study will accelerate mapping of vascular heterogeneity in vivo and, ultimately, catalyze progress toward development of affinity-guided smart drugs and contrast agents.
“In vivo phage display: identification of organ-specific peptides using deep sequencing and differential profiling across tissues”, Pleiko K, Põšnograjeva K, Haugas M, Paiste P, Tobi A, Kurm K, Riekstina U, Teesalu T; Nucleic Acids Research, (2021 Jan 14; https://doi.org/10.1093/nar/gkaa1279); “;
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkaa1279/6097687
Congratulations to Prakash Lingasamy for successfully defending his PhD thesis!
Prakash Lingasamy successfully defended his Ph.D. thesis " Development of multitargeted tumor penetrating peptides" on January 29th, 2021. Opponent of the thesis was professor Angelo Corti from Università Vita-Salute San Raffaele (Milan, Italy). Compared to conventional monospecific tumor homing peptides, multispecific peptides identified in the thesis provide a better accumulation of therapeutic and diagnostic nanoparticles in solid tumors. These preclinical results warrant downstream clinical studies on development of peptide-guided drugs and imaging agents. We wish Prakash good luck in his future career!
https://dspace.ut.ee/handle/10062/70738
https://dspace.ut.ee/bitstream/handle/10062/70738/lingasamy_prakash.pdf
Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity
Ludovico Cantuti-Castelvetri, Ravi Ojha, Liliana D Pedro, Minou Djannatian, Jonas Franz, Suvi Kuivanen, Franziska van der Meer, Katri Kallio, Tuğberk Kaya, Maria Anastasina, Teemu Smura, Lev Levanov, Leonora Szirovicza, Allan Tobi, Hannimari Kallio-Kokko, Pamela Österlund, Merja Joensuu, Frédéric A Meunier, Sarah J Butcher, Martin Sebastian Winkler, Brit Mollenhauer, Ari Helenius, Ozgun Gokce, Tambet Teesalu, Jussi Hepojoki, Olli Vapalahti, Christine Stadelmann, Giuseppe Balistreri, Mikael Simons.
Science 2020 Nov 13;370(6518):856-860.
doi:10.1126/science.abd2985. Epub 2020 Oct 20
Neuropilin-1 is a host factor for SARS-CoV-2 infection
James L Daly, Boris Simonetti, Katja Klein, Kai-En Chen, Maia Kavanagh Williamson, Carlos Antón-Plágaro, Deborah K Shoemark, Lorena Simón-Gracia, Michael Bauer, Reka Hollandi, Urs F Greber, Peter Horvath, Richard B Sessions, Ari Helenius, Julian A Hiscox, Tambet Teesalu, David A Matthews, Andrew D Davidson, Brett M Collins, Peter J Cullen, Yohei Yamauchi.
Science 2020 Nov 13;370(6518):861-865.
doi:10.1126/science.abd3072. Epub 2020 Oct 20
Two back-to-back collaborative studies published in Science on Neuropilin as a novel coronavirus receptor
Our lab was involved in two studies by international research teams that show that new coronavirus uses NRP-1 as a cellular internalization receptor.
The research stems from our earlier work on C-end Rule (CendR) peptides. CendR peptides act as position-dependent cellular switches: when present at C-terminus of any protein, they bind to Neuropilins to trigger cellular uptake and tissue penetration. The SARS-CoV-2 virus uses a protein called Spike to bind and enter host cells. New research shows that proteolytic processing of Spike protein by cellular enzyme furin results in activation of cryptic CendR peptide to trigger cellular uptake and tissue penetration of the CoV2 particles.
This discovery opens a new path for development of coronavirus drugs. Intriguingly, the CendR sequences are present on the surface of other pathogenic viruses, such as Ebola and HIV-1.
Our laboratory provided key reagents related to CendR pathway (antibodies, recombinant proteins, CendR nanoparticles) to the virology labs in Finland, UK, and Germany that coordinated the collaboration (PIs: Drs. Giuseppe Balistreri, Yohei Yamauchi, Mikael Simons and Peter J. Cullen).
LCB team included senior researcher Lorena Simón-Gracia, Ph.D. student Allan Tobi, and Prof. Tambet Teesalu.
Neuropilin-1 is a host factor for SARS-CoV-2 infection“ (DOI: 10.1126/science.abd3072)
Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity“ (DOI: 10.1126/science.abd2985)
Tumor penetrating peptide-functionalized Tenascin-C antibody for glioblastoma targeting
Lingasamy P, Laarmann AH, Teesalu T.
Curr Cancer Drug Targets. 2020 Oct 1.
doi: 10.2174/1568009620666201001112749. Online ahead of print. PMID: 33001014